Laboratory testing for COVID-19 has received a lot of attention during the current health crisis. By now, you may be wondering what type of testing, if any, is right for you and how you can access the testing you want.
In an interview on CNBC’s Squawk Box, Labcorp’s president and CEO, Adam Schechter explained, “There are two types of tests that Labcorp is performing for COVID-19. One that tells you if you have the virus [that causes COVID-19] and another test that tells you if you have had the virus in the past.”¹
As Schechter mentioned, there are key differences in these two types of coronavirus tests, and knowing more about the differences can help you better understand which COVID-19 test to consider for yourself or your family members.
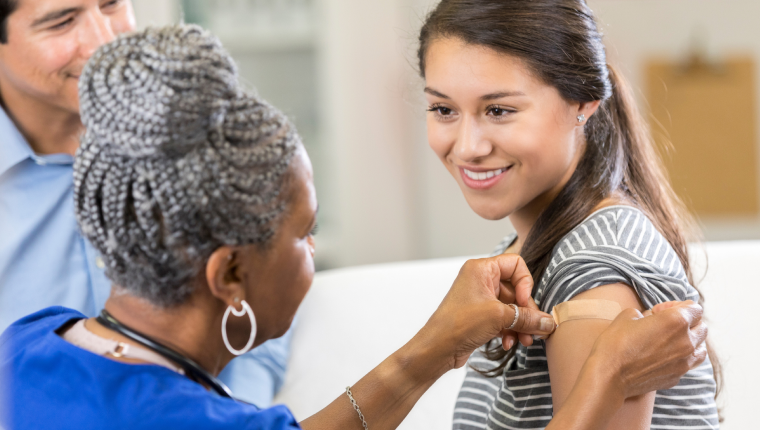
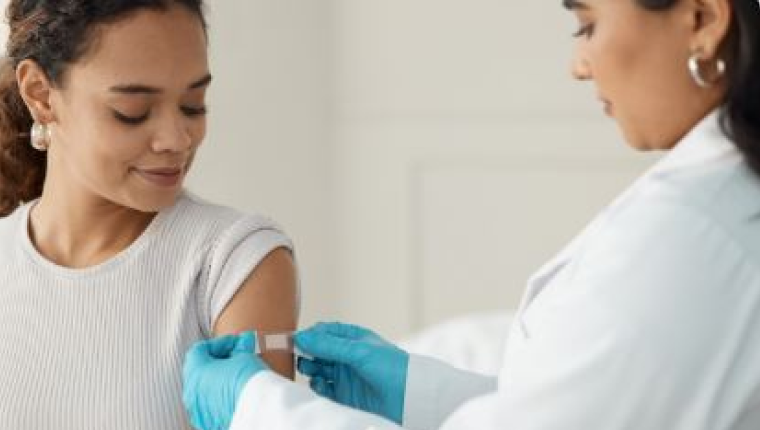
Detect Current Infection with a COVID-19 Swab Test
The COVID-19 molecular test, also known as a polymerase chain reaction (PCR) test, detects an active coronavirus infection. You may want to consider this test if you currently HAVE symptoms of COVID-19.
How can I get testing to see if I’m sick with COVID-19?
If you are experiencing symptoms consistent with an active COVID-19 infection, there are a couple of ways to access COVID-19 molecular testing.
- Healthcare Provider-Collected Sample: Labcorp, Labcorp OnDemand™’s parent company, launched a COVID-19 test on March 5, 2020, for ordering by authorized healthcare providers anywhere in the U.S.² You can access this test at your hospital or physician’s office where a trained healthcare provider can collect your sample.
- Self-Collected Sample: Another way to access this type of testing is with the at-home COVID-19 test kit available through Labcorp OnDemand. Labcorp received an Emergency Use Authorization (EUA)* from the U.S. Food and Drug Administration (FDA) that permits nasal swab specimens to be collected at home using the Labcorp OnDemand self-collection COVID-19 test kit.³ These test kits are available for consumers to order directly online after completing an eligibility survey.
How are samples collected and when do I get my results?
Both collection options use a swab to collect samples from your nose or throat as appropriate to the testing option chosen. Once your sample is collected, it is sent to an authorized laboratory testing facility for processing. If your sample was sent to a Labcorp lab, your results are made available 1-2 days after your sample is received by the lab.
Where do I find my results?
Healthcare Provider-Collected Sample: If your sample is collected by an authorized healthcare provider and sent to a Labcorp lab, your results will be made available to your provider through Labcorp Link™ and to you within the Labcorp Patient app. In addition, if you have trouble getting your COVID-19 results, you can submit the COVID-19 results inquiry form on Labcorp.com for more help.
Self-Collected Sample: If you used the Labcorp OnDemand at-home COVID-19 test kit to collect your sample, your results will be made available within your Labcorp OnDemand account (as well as to the ordering physician).
Detect Prior Infection with a COVID-19 Blood Test (Antibody)
What is an antibody test?
COVID-19 antibody tests, also known as serology testing, can check for different proteins (antibodies) that your immune system produces to fight SARS-CoV-2. It is possible to check for three different types of antibodies, known as IgM, IgA, and IgG. Of these three, IgG is the type that shows up later in the infection and might give you immunity to future SARS-CoV-2 infections.
This type of testing, when used to check for IgG, can detect a potential immune response to the virus. You may want to consider an antibody test if you HAD or THINK YOU HAD a COVID-19 infection and have been symptom-free for at least 1-2 weeks.
“While results from serological tests are neither the sole basis for diagnosis nor assurance of immunity, we believe the tests will play a critical role in helping healthcare providers determine appropriate treatment for individuals suspected of having been infected with the virus,” said Dr. Brian Caveney, chief medical officer and president of Labcorp Diagnostics.⁴
Important Note: This test has not been reviewed by the FDA. Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals. Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or inform infection status. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. SARS-CoV-2 serology testing is not intended as a primary diagnostic tool for COVID-19 nor as a sole basis for assurance of immunity.
How can I get COVID-19 Antibody Testing?
If you had or think you had a COVID-19 infection and are no longer symptomatic, you may consider talking to your healthcare provider about the COVID-19 antibody test.
The COVID-19 antibody test requires a blood sample that can be collected at your hospital, healthcare provider, or a Labcorp patient service center. Once your sample is collected it is sent to a laboratory testing facility for processing.
If your sample was collected at a Labcorp patient service center or sent to a Labcorp lab, your results will be made available within the Labcorp | Patient™ app.